Acids and Bases
Dublin Core
Title
Description
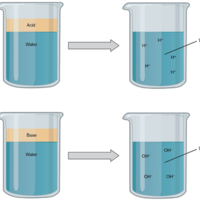
(a) In aqueous solution, an acid dissociates into hydrogen ions (H+) and anions. Nearly every molecule of a strong acid dissociates, producing a high concentration of H+. (b) In aqueous solution, a base dissociates into hydroxyl ions (OH–) and cations. Nearly every molecule of a strong base dissociates, producing a high concentration of OH–.
Contributor
Cut Rita Zahara
Rights
Creative Commons
Type
Files
Collection
Citation
“Acids and Bases,” Open Educational Resources (OER) , accessed March 1, 2026, https://oer.uinsyahada.ac.id/items/show/934.