Adenosine Triphosphate
Dublin Core
Title
Subject
Description
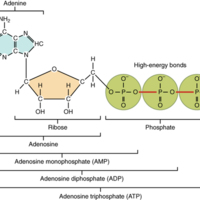
The nucleotide adenosine triphosphate (ATP), is composed of a ribose sugar, an adenine base, and three phosphate groups ([link]). ATP is classified as a high energy compound because the two covalent bonds linking its three phosphates store a significant amount of potential energy. In the body, the energy released from these high energy bonds helps fuel the body’s activities, from muscle contraction to the transport of substances in and out of cells to anabolic chemical reactions.
When a phosphate group is cleaved from ATP, the products are adenosine diphosphate (ADP) and inorganic phosphate (Pi). This hydrolysis reaction can be written:
ATP + H2O → ADP + Pi + energy
Removal of a second phosphate leaves adenosine monophosphate (AMP) and two phosphate groups. Again, these reactions also liberate the energy that had been stored in the phosphate-phosphate bonds. They are reversible, too, as when ADP undergoes phosphorylation. Phosphorylation is the addition of a phosphate group to an organic compound, in this case, resulting in ATP. In such cases, the same level of energy that had been released during hydrolysis must be reinvested to power dehydration synthesis.
Cells can also transfer a phosphate group from ATP to another organic compound. For example, when glucose first enters a cell, a phosphate group is transferred from ATP, forming glucose phosphate (C6H12O6—P) and ADP. Once glucose is phosphorylated in this way, it can be stored as glycogen or metabolized for immediate energy.
When a phosphate group is cleaved from ATP, the products are adenosine diphosphate (ADP) and inorganic phosphate (Pi). This hydrolysis reaction can be written:
ATP + H2O → ADP + Pi + energy
Removal of a second phosphate leaves adenosine monophosphate (AMP) and two phosphate groups. Again, these reactions also liberate the energy that had been stored in the phosphate-phosphate bonds. They are reversible, too, as when ADP undergoes phosphorylation. Phosphorylation is the addition of a phosphate group to an organic compound, in this case, resulting in ATP. In such cases, the same level of energy that had been released during hydrolysis must be reinvested to power dehydration synthesis.
Cells can also transfer a phosphate group from ATP to another organic compound. For example, when glucose first enters a cell, a phosphate group is transferred from ATP, forming glucose phosphate (C6H12O6—P) and ADP. Once glucose is phosphorylated in this way, it can be stored as glycogen or metabolized for immediate energy.
Contributor
Cut Rita Zahara
Rights
Creative Commons
Type
Files
Collection
Citation
“Adenosine Triphosphate,” Open Educational Resources (OER) , accessed March 4, 2026, https://oer.uinsyahada.ac.id/items/show/942.