Browse Items (27 total)
- Collection: 540 Chemistry & Allied Sciences
Properties and Chemistry of Benzene | Chemistry for All | The Fuse School
Tags: Chemistry, fuseschool
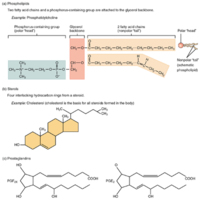
Other Important Lipids
Tags: Chemistry, Lipids, Phospholipids
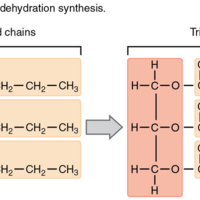
Triglycerides
Tags: Chemistry, Triglycerides
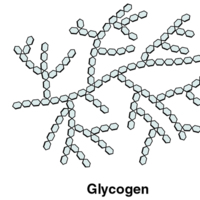
Polysaccharides
Starches are polymers of glucose. They occur in long chains called amylose or branched chains called amylopectin, both of which are…
Tags: anatomy, Chemistry, Polysaccharides
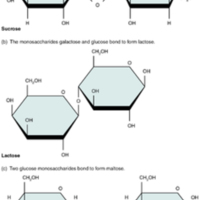
Disaccharides
Tags: anatomy, Chemistry, Disaccharides
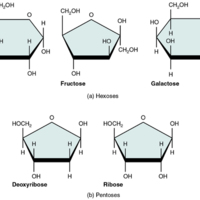
Monosaccharides
The remaining…
The Periodic Table of the Elements
Tags: Chemistry, Elements, Periodic Table, Science
Key Elements of Green Chemistry
Tags: Green Chemistry, Key Elements
Advances in Chemical Synthesis
compounds containing carbon. It is so important because millions of compounds that contain carbon are related to our daily lives, so organic chemistry has many…
Tags: Chemical Synthesis, Chemistry
Natural Resources Biometrics
Tags: Biometrics, Chemistry, Statistics
Chemistry
Tags: Chemistry
Self-Assembled Molecules – New Kind of Protein Ligands
penetrating in regions which are either naturally unstable or become temporarily accessible due…
General Chemistry
Tags: Chemistry, General Chemistry
Thermodynamics and Chemistry
Tags: Chemistry, Thermodynamics
Analytical Chemistry 2.0
Tags: Analytical, Chemistry
Concept Development Studies in Chemistry
Tags: Atom, Chemistry, Development Studies, Molecular
Chemistry Adventure
----------
Find us online!
Facebook:…
Tags: bubble science, catalase, catalyst, chemistry demo, chemistry video, classroom videos, decomposition, demo*, elephant toothpaste, Engineering, foam, h2, high school science videos, hydrogen peroxide, K-12, K12, K12 (Education), learning, M.I.T., middle school science videos, MIT, mit k12 videos, mit+k12, molecules, Science, science bubbles, science demo, science out loud, science videos, STEM, STEM videos, surface tension, teaching, videos