540 Chemistry & Allied Sciences
Dublin Core
Title
540 Chemistry & Allied Sciences
Items in the 540 Chemistry & Allied Sciences Collection
Properties and Chemistry of Benzene | Chemistry for All | The Fuse School
This video is part of 'Chemistry for All' - a Chemistry Education project by our Charity Fuse Foundation - the organisation behind The Fuse School. These videos can be used in a flipped classroom model or as a revision aid. Find our other Chemistry…
Chemical Process Dynamics and Controls
Process controls is a mixture between the statistics and engineering discipline that deals with the mechanism, architectures, and algorithms for controlling a process. Some examples of controlled processes are:
•Controlling the temperature of a…
•Controlling the temperature of a…
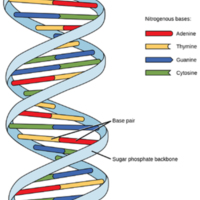
DNA
In the DNA double helix, two strands attach via hydrogen bonds between the bases of the component nucleotides.
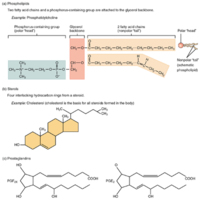
Other Important Lipids
(a) Phospholipids are composed of two fatty acids, glycerol, and a phosphate group. (b) Sterols are ring-shaped lipids. Shown here is cholesterol. (c) Prostaglandins are derived from unsaturated fatty acids. Prostaglandin E2 (PGE2) includes hydroxyl…
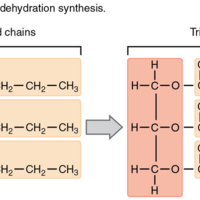
Triglycerides
Triglycerides are composed of glycerol attached to three fatty acids via dehydration synthesis. Notice that glycerol gives up a hydrogen atom, and the carboxyl groups on the fatty acids each give up a hydroxyl group.
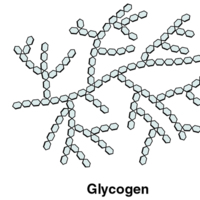
Polysaccharides
Polysaccharides can contain a few to a thousand or more monosaccharides. Three are important to the body:
Starches are polymers of glucose. They occur in long chains called amylose or branched chains called amylopectin, both of which are…
Starches are polymers of glucose. They occur in long chains called amylose or branched chains called amylopectin, both of which are…
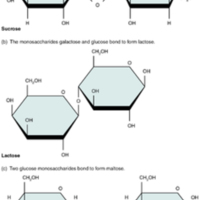
Disaccharides
A disaccharide is a pair of monosaccharides. Disaccharides are formed via dehydration synthesis, and the bond linking them is referred to as a glycosidic bond (glyco- = “sugar”). Three disaccharides are important to humans. These are sucrose,…
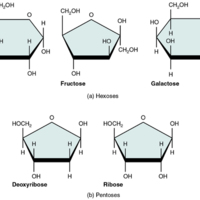
Monosaccharides
A monosaccharide is a monomer of carbohydrates. Five monosaccharides are important in the body. Three of these are the hexose sugars, so called because they each contain six atoms of carbon. These are glucose, fructose, and galactose.
The remaining…
The remaining…
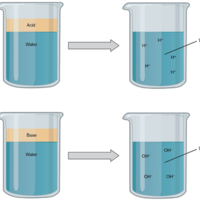
Acids and Bases
(a) In aqueous solution, an acid dissociates into hydrogen ions (H+) and anions. Nearly every molecule of a strong acid dissociates, producing a high concentration of H+. (b) In aqueous solution, a base dissociates into hydroxyl ions (OH–) and…
The Periodic Table of the Elements
Visit this website to view the periodic table. In the periodic table of the elements, elements in a single column have the same number of electrons that can participate in a chemical reaction. These electrons are known as “valence electrons.” For…
Key Elements of Green Chemistry
This document is a collaborative student work, comprising a directory of resources about mathematics and technology for kindergarten through fifth grade. This resource was created with the support of an ALG Textbook Transformation Grant. Topics…
Advances in Chemical Synthesis
Organic chemistry belongs to a branch of chemistry that is generally associated with
compounds containing carbon. It is so important because millions of compounds that contain carbon are related to our daily lives, so organic chemistry has many…
compounds containing carbon. It is so important because millions of compounds that contain carbon are related to our daily lives, so organic chemistry has many…
Natural Resources Biometrics
Natural Resources Biometrics begins with a review of descriptive statistics, estimation, and hypothesis testing. The following chapters cover one- and two-way analysis of variance (ANOVA), including multiple comparison methods and interaction…
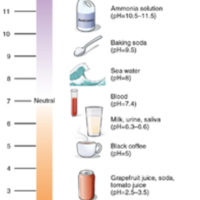
pH Scala
Open Educational Resource (OER) Unsyiah adalah satu satu Portal yang dikembangkan oleh Perpustakaan Universitas Syiah Kuala. OER Unsyiah berbasis Open Acces untuk siapa saja dan di mana saja. Bahkan Kami juga akan menerima kontribusi yang ingin ikut…
Fosforilasi Oksidatif dan Chemiosmosis
Open Educational Resource (OER) Unsyiah adalah satu satu Portal yang dikembangkan oleh Perpustakaan Universitas Syiah Kuala. OER Unsyiah berbasis Open Acces untuk siapa saja dan di mana saja. Bahkan Kami juga akan menerima kontribusi yang ingin ikut…
Chemistry
Chemistry is designed to meet the scope and sequence requirements of the two-semester general chemistry course. The textbook provides an important opportunity for students to learn the core concepts of chemistry and understand how those concepts…
Self-Assembled Molecules – New Kind of Protein Ligands
The standard substrate complexation mechanism engages natural binding sites. In contrast, supramolecular structures may form complexes with proteins by
penetrating in regions which are either naturally unstable or become temporarily accessible due…
penetrating in regions which are either naturally unstable or become temporarily accessible due…
Introductory Chemistry
David W. Ball of Cleveland State University brings his new survey of general chemistry text, Introductory Chemistry. This book is intended for a one-semester introductory or preparatory chemistry course. Throughout the chapters, David presents two…
General Chemistry
Wikibooks is for textbooks, annotated texts, instructional guides, and manuals. These materials can be used in a traditional classroom, an accredited or respected institution, a home-school environment, or for self-learning.
Thermodynamics and Chemistry
Thermodynamics and Chemistry is designed primarily as a textbook for a one-semester course in classical chemical thermodynamics at the graduate or undergraduate level. It can also serve as a supplementary text and thermodynamics reference source.
Analytical Chemistry 2.0
Analytical chemistry is more than a collection of analytical methods and an understanding of equilibrium chemistry; it is an approach to solving chemical problems. Although equilibrium chemistry and analytical methods are important, their coverage…
Concept Development Studies in Chemistry
Concept Development Studies in Chemistry is an on-line textbook for an Introductory General Chemistry course. Each module develops a central concept in Chemistry from experimental observations and inductive reasoning. This approach complements an…
Chemistry: Atoms First
Chemistry - Atoms First is a peer-reviewed, openly licensed introductory textbook produced through a collaborative publishing partnership between OpenStax and the University of Connecticut and UConn Student Government Association. This title is an…
Introductory Chemistry - 1st Canadian Edition
The goal of this textbook is not to make you an expert. True expertise in any field is a years-long endeavor. Here I will survey some of the basic topics of chemistry. This survey should give you enough knowledge to appreciate the impact of chemistry…
Chemistry Adventure
How do simple things like yeast, soap, and hydrogen peroxide come together to create a foam-splosion reaction? Tweak the ingredients to find out in this choose-your-own-adventure video series!
----------
Find us online!
Facebook:…
----------
Find us online!
Facebook:…
Collection Tree
- 500 Science
- 540 Chemistry
- 540 Chemistry & Allied Sciences
- 540 Chemistry